Guangdong medical device manufacturer with Class A quality credit
This month, Guangdong Food and Drug Administration announced on its official website that based on the relevant regulations of “Opinions on Supervision of Quality Credit Classification of Medical Device Manufacturers in Guangdong Province” (YSYJX [2011] No. 110) Lanmage was rated as a “Medical device manufacturer with Class A quality credit in Guangdong Province in 2016”.
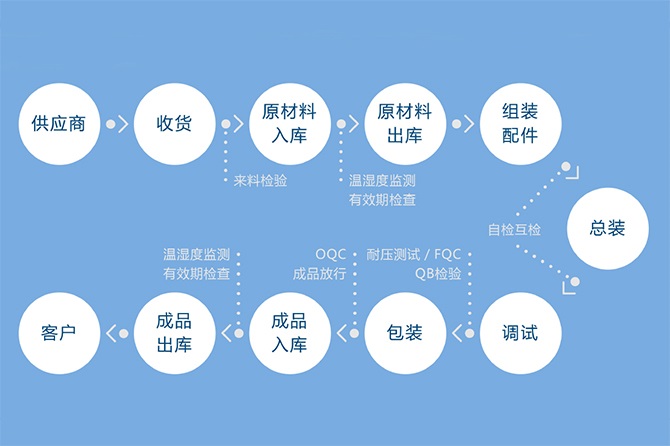
A medical device manufacturer with Class A quality credit refers to an enterprise with good quality management. This certification is only provided after strict assessment of corresponding indicators such as the enterprise’s medical device quality management system, product listing supervision sampling test conclusion, key items subject to daily supervision and inspection, and comprehensive quality management evaluation.
Lanmage was successfully rated as a “Medical device manufacturer with Class A quality credit”, which is thanks to our strict compliance with medical device-related management regulations and quality management system, innovative research and development, reliable quality, professional after-sales service and brand reputation.